中国组织工程研究 ›› 2015, Vol. 19 ›› Issue (8): 1267-1271.doi: 10.3969/j.issn.2095-4344.015.08.022
• 生物材料学术探讨 biomaterial academic discussion • 上一篇 下一篇
心血管支架材料生物相容性:国际发展态势的文献计量学分析
李 轩,石春来,王雪艳,张 明
- 辽宁省金秋医院心内科,辽宁省沈阳市 110016
Cardiovascular stent biocompatibility: bibliometric analysis of the international developmental trend
Li Xuan, Shi Chun-lai, Wang Xue-yan, Zhang Ming
- Department of Cardiology, Jinqiu Hospital of Liaoning Province, Shenyang 110016, Liaoning Province, China
摘要:
背景:心血管支架置入后血管再狭窄的确切发生机制尚不明确,减小置入后血管再狭窄的发生率就显得日益紧迫,置入后血管再狭窄除了与支架本身的抗腐蚀性、杨氏模量、屈服强度、材料柔顺性等生物力学有关外,还与置入后宿主的炎症反应、内皮细胞增殖、纤维组织细胞增生等生物相容性有关。
目的:以汤森路透Web of Science数据库及北美临床试验注册中心的信息为数据源,分析国际心血管支架材料生物相容性研究文献趋势,以期为该领域研究提供可借鉴的依据。
方法:①被收录的文章及以关键词“cardiovascular stent”(心血管支架),“biocompatibillty”(生物相容性) 进行检索。②检索文献时间范围10年(2005至2014年)。③检索信息源:Web of Science数据库和北美临床试验注册中心的临床试验项目。
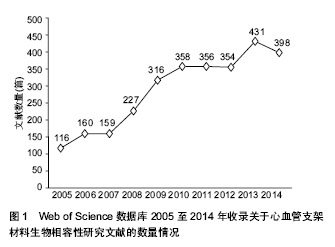
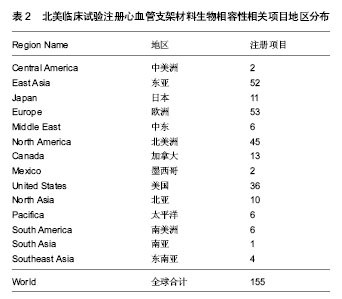
结果与结论:①以Web of Science数据库2005至2014年收录的心血管支架材料生物相容性相关的2 875篇文献为研究对象,利用Web of Science数据库文献分析报告以及导出信息功能进行文献分析,得到8篇被引频次较高的经典文献。②在汤森路透Web of Science数据库,2005至2014年关于心血管支架材料生物相容性发表的文献共2 875篇。美国发文量最多,931篇,占总数比重最大,为32.383%;2005至2014年发表心血管支架材料生物相容性的研究文献较多的机构是美国哈佛大学,美国布莱根妇女医院,奥地利维也纳医科大学,荷兰鹿特丹伊拉斯姆斯大学,美国哥伦比亚大学;American Journal of Cardiology (《美国心脏病学杂志》)发表此领域文献最多,117篇,占全部文献的4.070%;近年来,国际心血管支架材料生物相容性相关文献呈总体逐渐上升的趋势;2005至2014年,在2 875篇心血管支架材料生物相容性的研究文献中来自中国的文章有223篇,文章数量位居第4,说明中国在此领域的科研成果较多。③2005至2014年,在北美临床试验注册中心注册的心血管支架材料生物相容性相关临床试验注册项目共有155项,其中干预性研究有140项,观察性研究有15项。
中图分类号: